Abstract
Mantle cell lymphoma (MCL) is an aggressive B cell malignancy that is not yet curable. Ibrutinib was approved in 2013 for the treatment of relapsed/refractory MCL patients; however, ibrutinib resistance inevitably develops. Therefore, there is an urgent unmet need to overcome ibrutinib resistance and to study alternative treatment options.
Constitutive NF-κB activation is the hallmark of MCL. Indeed, next generation sequencing analysis of 110 MCL patient samples revealed that genes belonging to the NF-κB signaling pathway had a high mutation rate (28.8%), indicating a significant contribution of NF-κB signaling to MCL malignancy. Mucosa-associated lymphoid tissue transformation protein (MALT1) plays a crucial role in NF-κB signaling. MALT1 is a unique paracaspase within the human genome, and the proteolytic activity of MALT1 has been found to be constitutively active in many MCL samples, suggesting that MALT1 may be a potential therapeutic target without significant off-target side effects. MI-2 is a specific inhibitor of MALT1 and its safety and efficacy are currently being examined in a clinical trial for the ABC subtype of diffuse large B cell lymphoma. In addition, animal models treated with MI-2 did not have any detectable physiological, histological or biochemical signs of toxicity. However, whether MALT1 activity contributes to ibrutinib resistance and whether targeting MALT1 can overcome ibrutinib resistance in relapsed/refractory MCL patients remain unclear.
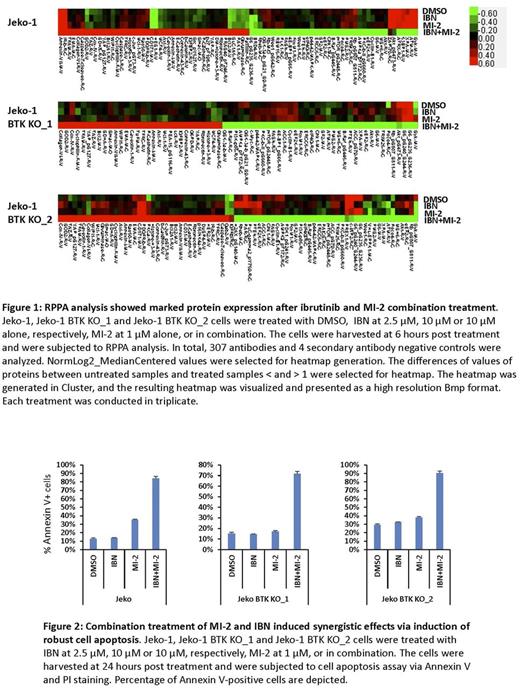
In this study, we found that both canonical and non-canonical NF-κB signaling is activated in ibrutinib-resistant MCL cells, which correlates with constitutive MALT1 activity. Interestingly, we found that MALT1 is highly mutated in four clusters including the death domain, TRAF6 binding site, Caspase like domain, and IKKγ binding site. Occurrence of L79P, K80R, E319D, L445P and N446S strongly correlated with ibrutinib resistance and disease progression. Treatment with MI-2 at nanomolar levels significantly reduced cell viability and induced apoptosis in several MCL cell lines. MI-2 treatment inhibited NF-κB activation, IL-6 production and downstream STAT3 activation. Combination of MI-2 and ibrutinib resulted in synergistic growth inhibition in both ibrutinib-resistant MCL cell lines and primary MCL cells. RPPA analysis showed that treatment with the MI-2 and ibrutinib combination resulted in marked changes in the cellular protein profiles, suggesting that targeting MALT1 catalytic activity in MCL is a promising therapy to overcome ibrutinib resistance in relapsed/refractory MCL patients. Furthermore, MI-2 treatment greatly inhibited the in vitro cell migration of the majority of examined MCL cell lines as well as cell adhesion to the extracellular matrix, including integrin and laminin; therefore, treatment with MI-2 may induce lymphocytosis (i.e., compartmental shift) in patients and mouse xenograft models.
Altogether, our data suggest that targeting MALT1 is a very promising therapy for treating relapsed/refractory MCL patients. These studies support the development of preclinical and clinical studies to overcome ibrutinib resistance by targeting MALT1.
Wang: Proteolix: Honoraria, Research Funding; Pharmacyclics: Research Funding; Onyx: Research Funding; Kite Pharma: Research Funding; Juno Therapeutics: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Dava Oncology: Honoraria; Celgene: Honoraria, Research Funding; BeiGene: Research Funding; Asana Biosciences: Research Funding; Acerta Pharma: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.